Improvements in image resolution, field of view, and focal length in today’s smaller endoscopes are helping clinicians perform biopsies, insert stents, and staple wounds.
RICHARD YANG, OMNIVISION TECHNOLOGIES INC.
Many patients would prefer to undergo surgery in which tools enter their bodies through a natural opening or small incision, over an operation involving the opening of their chests or abdominal cavities. Improvements in medical imaging over the past few decades have allowed for many procedures that use the former method. Endoscopy is the technique that has enabled doctors and their patients to make this choice.
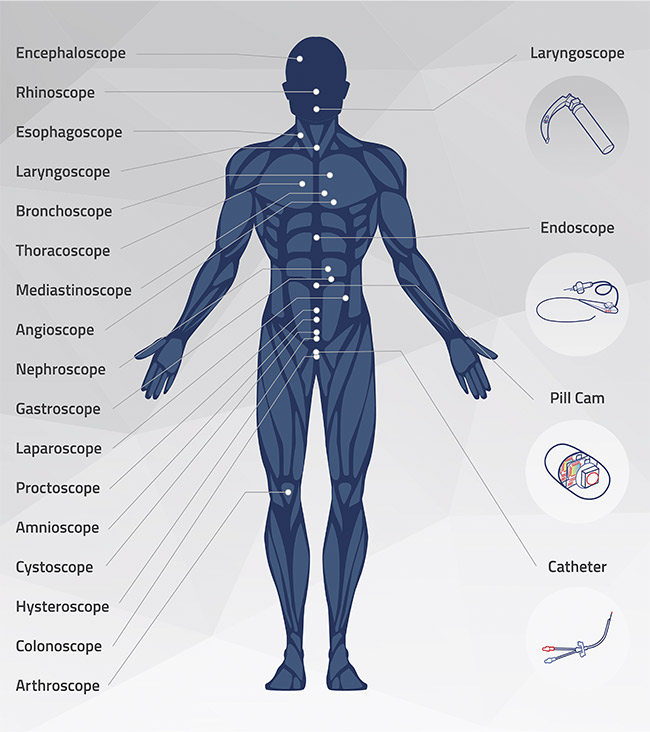
Different types of endoscopes target specific areas of the human body. Courtesy of OmniVision.
Many types of medical imaging — including x-rays, ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) — allow doctors to diagnose and treat a wide variety of medical conditions. But endoscopy is unique among the less-invasive approaches in that it improves outcomes and reduces patient discomfort, because it enables in situ diagnosis and therapy and minimally invasive surgery (Figure 1). Endoscopes on the high end of the market have advanced, with increasing applications in all aspects of patient care in the hospital setting. And endoscopes on the low end of the market have also been enhanced, with the recent development of simplified tools that can be used by smaller clinics to perform more routine, less-invasive diagnostic procedures.
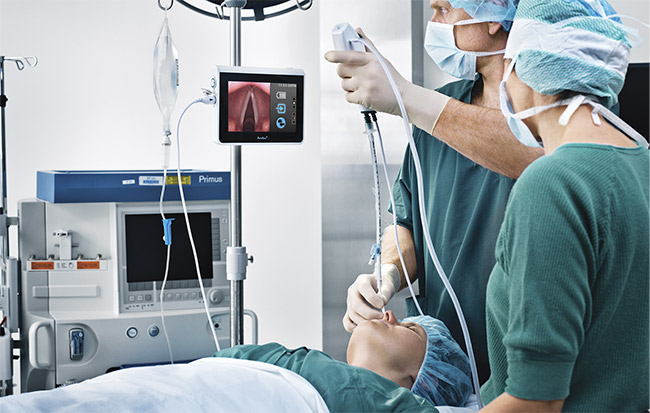
Figure 1. Doctors use an endoscope to intubate a patient. Courtesy of Ambu A/S.
Different types of endoscopes target different parts of the body to provide a view inside nearly every hollow organ (see image at beginning of article). Common applications of these diagnostic procedures include ear, nose, and throat; airway management; gastroenterology; ureteroscopy; gynecology; arthroscopy; and cardiology. Refinements in endoscope design and components are making endoscopy accessible for a greater variety of procedures than ever before. These advancements are changing the market for endoscopes, not only in terms of the applications served, but also by allowing for disposable instruments.
Market trends
As the average age of the world’s population increases, so too does the incidence of conditions made more likely by this longevity — such as hypertension, heart failure, and many types of cancer. Early diagnosis gives doctors more options for treatment and improves prognoses for patients. But more testing can drive up costs, and health care providers are under pressure to conduct procedures as quickly as possible. The challenge is to accomplish this without sacrificing quality of care. Diagnosis and treatment must be both efficient and accurate.
A growing number of hospitals are investing in endoscopy because the technique reduces risk via better diagnostics that are enabled by direct imaging of an affected area. Endoscopy is also minimally invasive, which lowers costs by shortening hospital stays, compared to other diagnostic methods and treatment options. Governments around the world are directing research dollars toward advancements in endoscopy, accelerating the pace of new technology development. In the U.S., health care insurance is also more likely to reimburse hospitals for endoscopic procedures, making such procedures more affordable for patients.
The transition of medicine from hospitals to clinics, and eventually to the home, also supports a greater need for available endoscopic technology. Moving common procedures out of hospitals tends to make the procedures both less expensive and more accessible. But many types of medical imaging require large, expensive equipment that will not fit in a clinical setting and that is beyond the budget of independent medical practices. Technology advancements allow for portability (as opposed to the larger, older endoscopes that use tower-based camera control and video processing units), making newer endoscopes ideally suited for use away from hospitals and in smaller medical clinics. While the devices are not currently being used in home care settings, many research programs are looking into using endoscopes for remote patient monitoring.
Technological challenges
Endoscopy can meet the need for faster diagnosis, but the technology is not without inherent challenges. Not all endoscopes offer sufficient resolution and image clarity to provide accurate diagnoses and facilitate the successful surgical outcomes that medical professionals and patients expect. Unless users can trust the results, they will be less likely to rely on endoscopy and may instead resort to additional tests that increase costs or more invasive procedures that drive up both cost and risk.
Compared to technologies that offer direct imaging that “sees” the operating field just as the doctor sees it during open surgery, legacy endoscope technologies do not enable doctors to see exactly where they are cutting or clamping as they work. Doctors must instead rely on using other imaging technologies — x-ray or ultrasound, for example — in parallel with legacy endoscopy to complete a procedure successfully.
Beyond the resolution challenges, human hands are less precise than the moving parts of machines. Robotic surgery is an increasingly attractive option for medical procedures because the instruments are much more flexible and dexterous than a surgeon’s hand or wrist (Figure 2). Such instruments can rotate a full 360° and be directed to pinpoint an exact location. However, to successfully achieve better procedural outcomes, robotic surgery requires integration with real-time, high-resolution machine vision capabilities and intuitive human-machine interfaces.

Figure 2. Endoscopic instruments for robotic surgery. Courtesy of Intuitive Surgical Inc.
Evolving solutions
Endoscopes have evolved since the introduction several decades ago of cameras that use charge-coupled device (CCD) image sensors. The key device parameters — image resolution, field of view, focal length, and device size — keep improving in development. The clearer the image, the wider the field of view, and the more precise the sense of depth, the better the outcomes of procedures for patients. The smaller the device, the deeper it can go into the body and the greater variety of organs it can reach.
Incorporating surgical tools into endoscopes enables diagnosis and treatment at the same time. Removal of polyps during colonoscopy is one example of how this synergy works. As tools become smaller and imaging capabilities increase, applications for use continue to expand.
The vast array of applications makes endoscopes a broad product category. More than 30 types of endoscopy exist, and some procedures require over a dozen different endoscopic tools. How can designers make components for all of these varied devices? The solution lies in dividing the market into a small number of categories and providing multipurpose components that offer a turnkey solution. Integrating a medical-grade CMOS image sensor (CIS) into the camera module meets this need.
In the past, improving both resolution and device size at the same time was not a reasonable goal. The introduction of miniature CISs, which can be fabricated in high volume on silicon wafers, has changed the specifications of components in the manufacturing of endoscopes. High resolution, small size, and low cost are no longer mutually exclusive in this formula.
Rigid or flexible endoscopes consisting of a glass rod lens and a CCD-based camera have long been the industry standard. These devices are now often being replaced with CIS-based endoscopes. Instead of placing the camera inside the handle, as is done with CCD technology, the small size of CISs allows cameras to be located at the endoscope tip — known as a “chip-on-the-tip” design. Locating the image sensor on the tip instead of the handle opens up endless possibilities for the endoscope’s use.
Seeing deep inside the body
The most exciting opportunities hinge on this integration of image sensors directly into the tips of endoscopic tools. Procedures that were previously performed “blind” — relying only on indirect imaging technologies such as x-ray or ultrasound — can now take advantage of advanced endoscopes to improve accuracy and reduce the duration of surgery by showing the exact area where an operation is occurring. Applications include needle biopsies, stents, surgical clips, wound stapling, and vein harvesting. Focal lengths down to 2 mm, combined with a wide field of view, allow doctors to directly visualize the area of interest in 3D.
Chip-on-the-tip endoscopes bring multiple advantages. A CIS can achieve extremely high resolution, matching that of CCD-based cameras. Resolutions of 720 and 1080 pixels, and even 4K2K, are now available, resulting in the clear images needed for the most accurate diagnoses. Optics with a short focusing distance of 2 to 50 mm provide accurate 3D imaging for these purposes. A medical CIS may also provide a high dynamic range capability, which expands the color range, producing greater contrast between light and dark regions.
The smallest commercially available image sensor in the world, according to Guinness World Records1, measures less than 0.6 mm on a side (Figure 3). The CMOS manufacturing process drives down the cost of producing these image sensors, since tens of thousands of sensors are fabricated in parallel on a single wafer. Additionally, the smallest medical cameras have optics that are fabricated at the wafer level as well, and are bonded to the sensor to form a camera module that is less than 1 mm high. These wafer-level camera modules can fit onto the tip of a disposable endoscope with a 1-mm diameter.

Figure 3. The world’s smallest medical-grade CMOS image sensor. Courtesy of OmniVision.
Wafer-level cameras are so small that designers can embed them directly into the functional end of endoscopic tools such as forceps and scissors (Figure 4). Doing so gives doctors a 3D view during biopsies or other endoscopic surgery applications. High-frame-rate video — up to 60 fps — provides doctors with accurate real-time images that are projected to a screen located in the operating room.
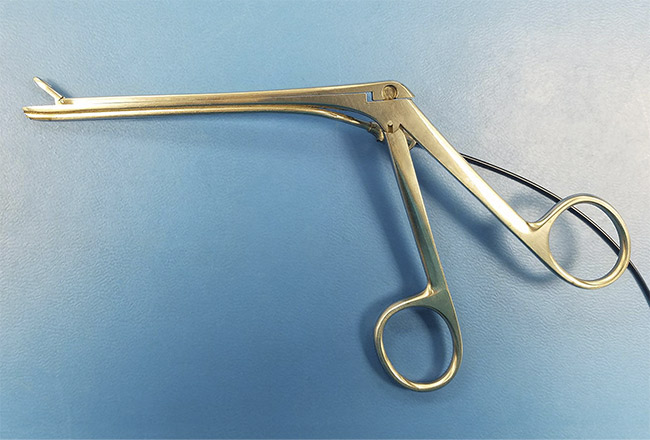
Figure 4. Endoscopic forceps with an embedded wafer-level camera. Courtesy of Myriad Fiber
Imaging Tech. Inc.
Tiny camera modules are also invaluable for advancing robotic surgery. This type of surgery has been used for laparoscopic procedures for two decades, but the extremely small size and high resolution of the latest generation of medical-grade CISs, plus advancements in computer integration, have propelled the technique forward. In recent years, more medical device companies have entered the market, thereby expanding the variety of approved medical procedures possible. Orthopedic, cardiac, urogenital, and cranial surgeries can all be performed using surgeon-controlled robotic tools.
In addition, the low cost of CISs allows cameras to be integrated into disposable, single-use endoscopes. This function eliminates the cross-contamination risk that reusable devices pose.
Cross-contamination
Cross-contamination is a serious concern for both doctors and patients because endoscopes enter body cavities and are potentially exposed to infectious and transmissible diseases. Reusable devices are cleaned between patients, but not all facilities reprocess the endoscopes according to the most stringent specifications. The protocols for cleaning and disinfecting, or sterilizing the devices are quite involved, and staff are under intense time pressure to complete a variety of tasks in addition to cleaning. Infection risk following substandard sterilization of reusable devices ranks third on the Emergency Care Research Institute’s list of Top 10 Health Technology Hazards for 20202. While increased attention to cleaning procedures will help, it is extremely difficult to ensure compliance throughout the health care industry. Disposable endoscopes will help alleviate this need.
As a current case in point, the COVID-19 pandemic is exacerbating the long-existing problem of cross-contamination in the hospital setting3. If reusable tools are not thoroughly cleaned, the virus can be transferred between patients. Medical staff are also at risk of exposure during the cleaning process.
Putting patients on ventilators is an especially risky procedure from the viewpoint of potential employee exposure to the virus. Laryngoscopy is used during intubation, before placing patients on ventilators, to improve the success rate of the procedure (Figure 5). Conducting this procedure via video allows medical personnel to maintain a safe distance between themselves and the patient, reducing risk of exposure to the virus. For precautionary reasons, single-use CIS-based endoscopes are used extensively for COVID-19 patients. Disposable bronchoscopes are also in high demand in hospitals worldwide for diagnosing lung damage in patients with confirmed or suspected COVID-194.
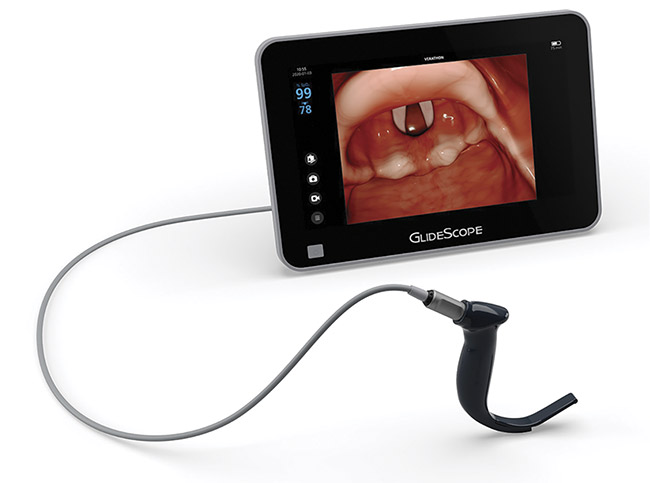
Figure 5. Laryngoscopes improve intubation for COVID-19 patients. Courtesy of Verathon Inc.
The future of endoscopy
Technological advancements and cost reductions have expanded possible applications for endoscopy, but further improvements are coming from the production pipeline. By installing the camera in the tip of the device rather than in the endoscope handle, the size of the components is reduced and thus the cost of endoscopic tools is dropping, and so they are becoming more universally available. Additionally, the high-volume CIS chip production that is made possible in semiconductor fabrication facilities leads to higher-resolution image sensors without increasing cost.
Endoscopy currently operates in the visible region of the electromagnetic spectrum. But image sensors are not restricted to the wavelengths of light, between 400 and 700 nm, that are visible to the human eye. Expanding into the near-infrared (NIR) region (Figure 6) offers unique advantages, especially for diagnosing and treating cancer cells. It also enables machine vision for a broad range of anatomy, blood vessel, and structure detection applications.
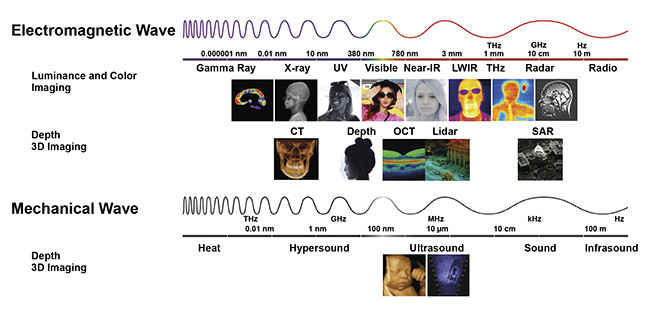
Figure 6. Imaging is the ability to ‘perceive’ the environment at a distance using propagating waves. An image can be perceived if a matrix of
at least 50 × 50 pixels is received. Endoscopy is expanding from the visible into the near-infrared portion of the electromagnetic spectrum.
Courtesy of Yole Développement.
NIR-capable CMOS image sensors are already in commercial use, in applications such as security, allowing cameras to detect intruders at night. Medical-grade CISs that are capable of capturing images at the wavelengths of 860 or 940 nm using an NIR light source are also available, enabling more precise targeting of tumors and in situ cancer treatment, as well as machine vision for anatomy and structure detection. In fact, studies have already demonstrated the promise of NIR-enabled immunotherapies as a method of killing cancer cells in patients with inoperable tumors5.
Additionally, combining fluorescence with NIR imaging can enable endoscopy to be used in conjunction with nuclear medicine. In patients who have been injected with radioactive tracers, affected areas will reflect IR light differently, allowing for even greater precision when diagnosing or treating tumors imaged by endoscopes.
The trend toward telemedicine and remote patient monitoring is another potential avenue for expanding the use of endoscopy. Camera capsules are only one possible at-home application for CISs. In these capsule endoscopy or “pill cam” procedures, the patient swallows a capsule containing a wireless camera that takes pictures as it travels through the digestive tract. The camera then transmits the pictures to an external recorder before being expelled from the body. In the future, examination tools to detect ear or sinus infections could take the form of simple, inexpensive endoscope-like devices. Patients could pick up prescription devices at a pharmacy, insert them into their ear, nose, or mouth at home, and have the results sent remotely to a doctor for evaluation.
Multiple trends in health care are converging to support the greater adoption of endoscopy. Chip-on-the-tip endoscopic tools with integrated CMOS image sensors are well positioned to provide for new applications. The technology keeps improving, with higher image resolution, greater control over focal length, wider field of view, a broader range of frequencies for imaging, device sizes that are shrinking, and overall reduced cost. Greater integration of image sensors and signal processing will lead to greater adoption of robotic surgery and the expansion of endoscopy into medical clinics and, eventually, into our homes.
Meet the author
Richard Yang joined OmniVision Technologies Inc. in 2019 as a senior staff product marketing manager. He is responsible for marketing and business development within the company’s medical segment. Prior to joining OmniVision, Yang was with Altera (acquired by Intel), in a number of product marketing roles, including leadership in driving business success for its medical segment. He received an MBA from Simon Fraser University and a Bachelor of Science in electrical engineering from the University of British Columbia in Vancouver; email: [email protected].
References
1. Guinness World Records (2019). Smallest commercially available image sensor, www.guinnessworldrecords.com/world-records/493752-smallest-commercially-available-image-sensor.
2. ECRI Institute (2020). Top 10 health technology hazards for 2020: expert insights from health devices. Executive brief, https://elautoclave.files.wordpress.com/2019/10/ecri-top-10-technology-hazards-2020.pdf.
3. C.L. Ofstead et al. (2020). Potential impact of contaminated bronchoscopes on novel coronavirus disease (COVID-19) patients. Infect Control Hosp Epidemiol, Vol. 41,
Issue 7, pp. 862-864.
4. American Association for Bronchology and Interventional Pulmonology (AABIP) (2020). Statement on the use of broncho-scopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection, www.doi.org/10.1097/lbr.0000000000000681.
5. K. Sato et al. (2018). Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: a mechanism of cancer cell cytotoxicity after near-infrared photoimmunotherapy. ACS Cent Sci, Vol. 4, Issue 11, pp. 1559-1569.